Hbro3 Strong or Weak Acid or Base
The pKa of HBrO3 is -2 and of HBr04 is -46. The easiest way to do this is just memorize the 7 strong acids and 8 strong bases.

Aleks Understanding The Difference Between Strong And Weak Acids Youtube
There are two ways to determine whether HI is a strong or weak acid.

. Lets put some numbers on this. The conjugate base of HCN is Cyanide CN. For general chemistry courses t.
The following are some less common acids that are also strong. Electronegativity is the ability of an atom to pull electrons towards itself. Is bromate harmful to humans.
Learn vocabulary terms and more with flashcards games and other study tools. It is a bromine oxoanion and a monovalent inorganic anion. Strong acid Weak acid Strong base Weak base Answer Bank H2CO3 KOH NH3 HBr H3PO4 HSO4 Ba OH2.
As you see in the above reaction NH3 is a weak base and we know a weak base always forms a conjugate acid not necessarily the strong one. Is phosphoric acid strong or weak acid. In chemistry neutralization or neutralisation see spelling differences is a chemical reaction in which an acid and a base react quantitatively with each other.
The strong bases are NaOH KaOH CsOH CanOH2 SrOH2 RbOH BaOH2. Click to see full answer. Hypobromous acid is a weak unstable acid with the chemical formula HBrO where the bromine atom is in the 1.
Hydrobromic acid formula HBr. HClO 3 HBrO 3 HIO 3 H 2 SeO 4 Assume all other acids are weak unless told otherwise. However HCN is a weak acid due to only partial dissociation of its ions in an aqueous solution also the dissociation constant K a for HCN is 62 10 -10 which is considered far low for the strong acid.
HBrO4 is a stronger acid than HBrO3 by. Chemistry questions and answers. Acid strength order.
Correct option is A ClBrI all belong to the same group. During a reaction with a strong base the weak acid is forced to completely dissociate there is no weak acid molecule left intact at the equivalence point. Hno 3 Hcl Hbr Hi H 2 So 4 And Hclo 4 Are The Strong Acids Strong And Weak Acids Bases The Strength Of An Acid Or Base Is Determined By The Amount Ppt Download The pKa of HBrO3 is -2 and of HBr04 is -46.
As we discussed earlier NH 3 is a weak base hence it will form a conjugate acid by adding one proton to itself. LIST ACID NH4ClO4 NH4Cl HBrO WEAK H2PO4-H3PO3 WEAK HNO3 STRONG HCl STRONG H2S WEAK H2SO4 STRONG H3PO4 WEAK H2CO3 WEAK HBr STRONG HI STRONG HClO4 STRONG HClO3. Hydrofluoric Acid HF weak acid.
HBraq OH-aq H201 Braq A HBO B OH C H20 D Br 5. All ionic hydroxides are strong bases regardless of solubility. Hypochlorous acid formula HClO.
Identify the conjugate base in the following reaction. HF HNO 2 HClO 2 H 2 SO 3 SO 2 H 2 O HC 2 H 3 O 2 HOAc 2. A strong acid B weak acid C impossible to tell 6.
Hydroiodic acid formula HI. So no since HBrO3 and HBrO4 dont completely dissociate they are not considered strong acids. However the accepted value for the Hrxn for CH3COOH is about 19 kJmol less than for the HCl.
This means that both are strong acids completely ionized in water. Because it is a weak acid its conjugate base OBr- when in a salt form with a metal such as Na giving NaOBr which is a weak base and would produce an alkaline basic solution in water. Bromic acid formula HBrO3.
Based on this information is HBr a strong acid or weak acid. Strong acid Weak acid Strong base Weak base Answer. If you dissolve 0025 mol of HBr in 1 L of water the resulting solution contains 0025 mol of H30.
The strong acids are HCl HBr HI HNO3 HClO4 HClO3 H2SO4 H2SeO4. Consequently is NaOBr an acid or base. It is a conjugate base of a hypobromous acid.
HCN is acting as an Arrhenius acid and Bronsted-Lowry acid. HCl is a strong acid and CH3COOH is a weak acid. Cancer Hazard Potassium Bromate may be a CARCINOGEN in humans since it has been shown to cause kidney thyroid and gastrointestinal cancer in animals.
Classify the compounds as a strong acid weak acid strong base or weak base. The first it to memorize the seven common strong acids. Because it is formed from the reaction of a strong base MgOH2 and a.
Start studying Strong and weak acids and bases. Bases that do not contain OH are weak. As one goes down the group the atomic size increases and electronegativity decreases.
Chlorous acid formula HClO2. Chloric acid formula HClO3. Classify the compounds as a strong acid weak acid strong base or weak base.
It is weak acid. Yes it is a salt but when hydolyzed in water it will have a pH that is slightly basic. Anything else is weak pretty much.
Perchloric acid formula HClO4. Learn vocabulary terms and more with flashcards games and other study tools. Start studying Strong and Weak Acids and Bases.
Hydrochloric acid formula HCl. HBrO3 only exists in solution but is a strong acid so its completely dissociated into Haq and BrO3-aq.
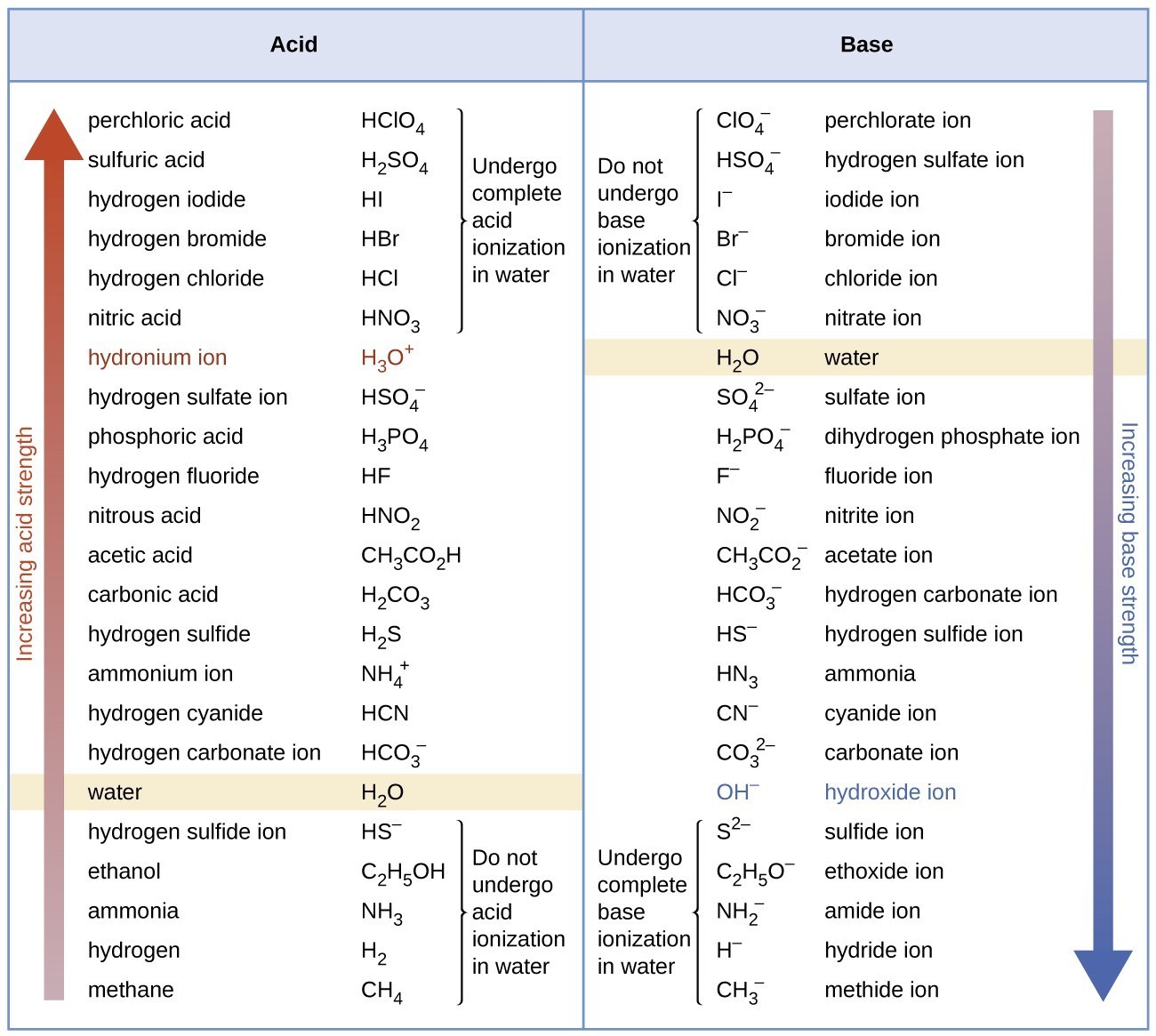
14 3 Percent Ionization And Relative Strengths Of Acids And Bases Chemistry

Strong Acids And Bases Mcat Chemistry Cheat Sheet Study Guide Studypk Chemistry Lessons Teaching Chemistry Chemistry Classroom

Hno 3 Hcl Hbr Hi H 2 So 4 And Hclo 4 Are The Strong Acids Strong And Weak Acids Bases The Strength Of An Acid Or Base Is Determined By The Amount Ppt Download
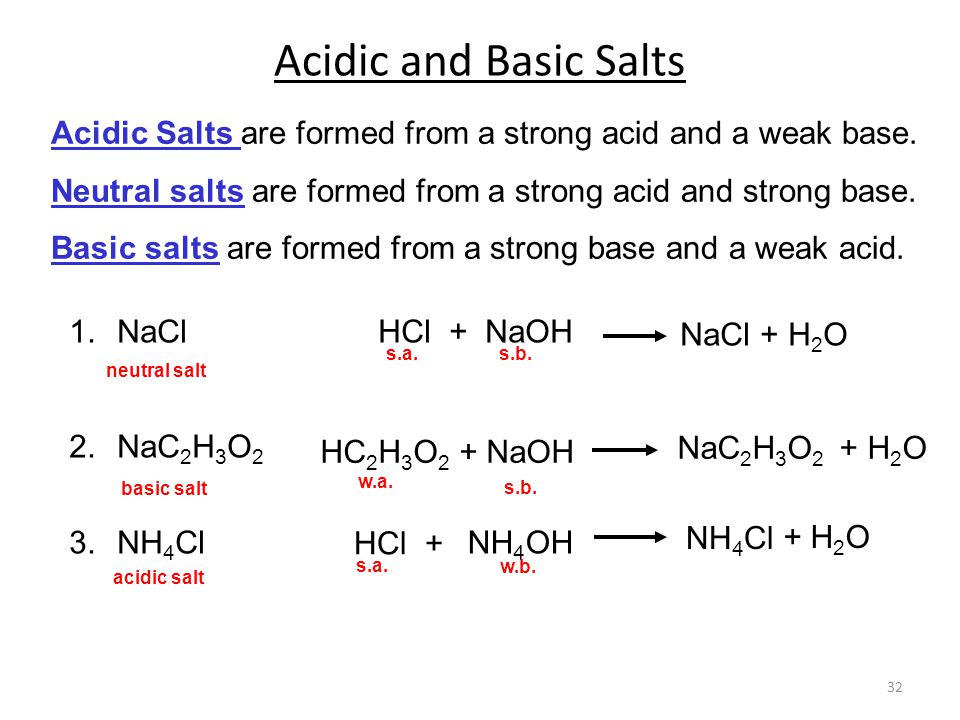
Chapter Ppt Video Online Download

Ppt Acids And Bases Salts And Solutions Powerpoint Presentation Free Download Id 368814

Strong And Weak Acids And Bases Green Damjii Chapter 8 Section 3 Chang Chapter 15 Copyright C The Mcgraw Hill Companies Inc Permission Required Ppt Download
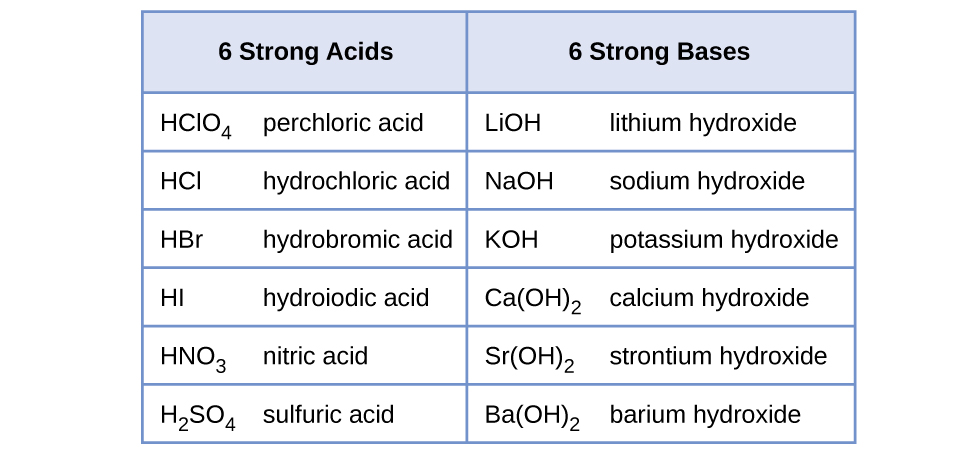
Relative Strengths Of Acids And Bases Chemistry 2e
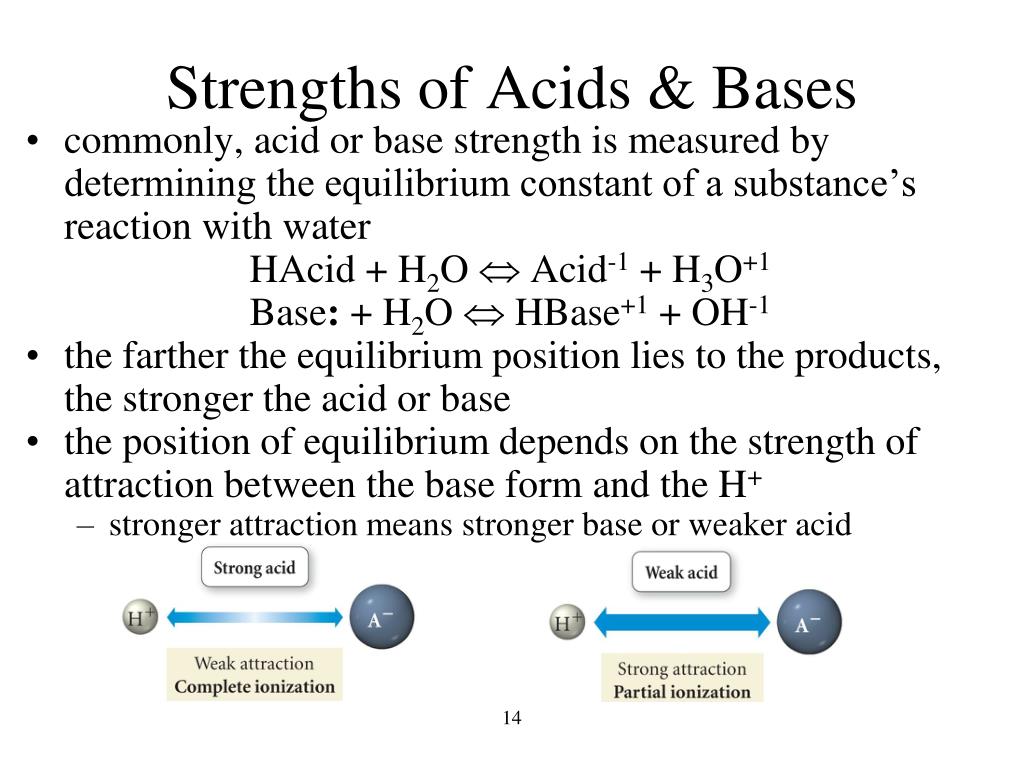
Ppt Chapter 15 Acids And Bases Powerpoint Presentation Free Download Id 792306

8 2 Weak Acids Bases Ionization Constants Percent Ionization For Weak Acids Most Weak Acids Ionize 50 Percent Ionization P General Weak Acid Ppt Download
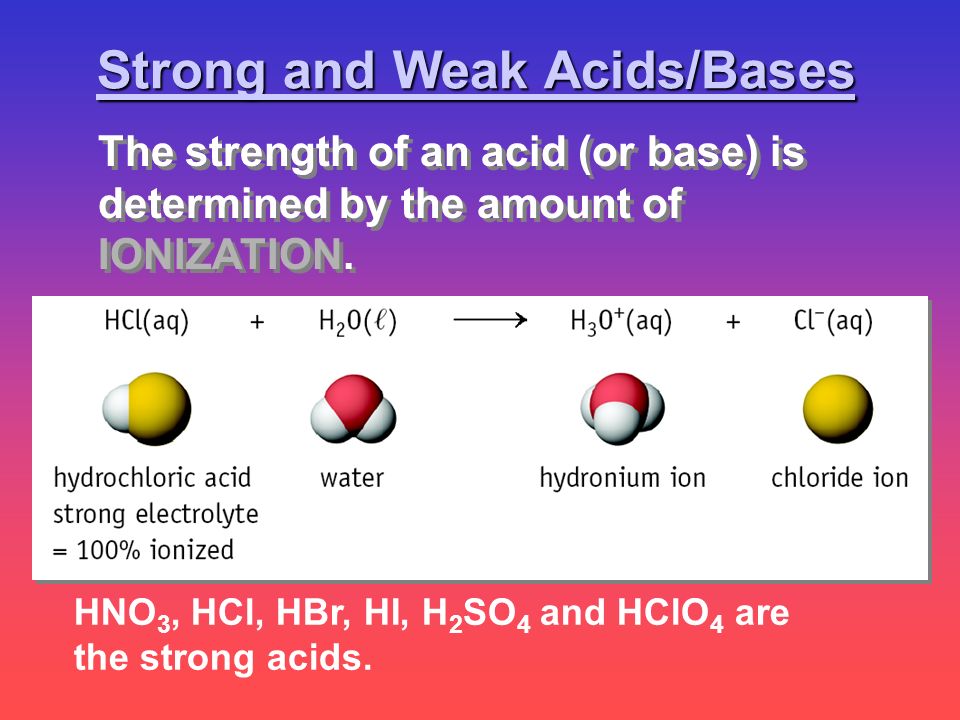
Hno 3 Hcl Hbr Hi H 2 So 4 And Hclo 4 Are The Strong Acids Strong And Weak Acids Bases The Strength Of An Acid Or Base Is Determined By The Amount Ppt Download

Strong Versus Weak Acids And Bases

Aleks Understanding The Difference Between Strong And Weak Acids Youtube

List Of Strong Weak Acids Bases

Strong Acids And Strong Bases Acids And Bases That Are Strong Electrolytes Completely Ionized Chemistry Education Teaching Chemistry Organic Chemistry Study

How To Determine If Acid Is Strong Or Weak Shortcut W Examples And Practice Problems Youtube
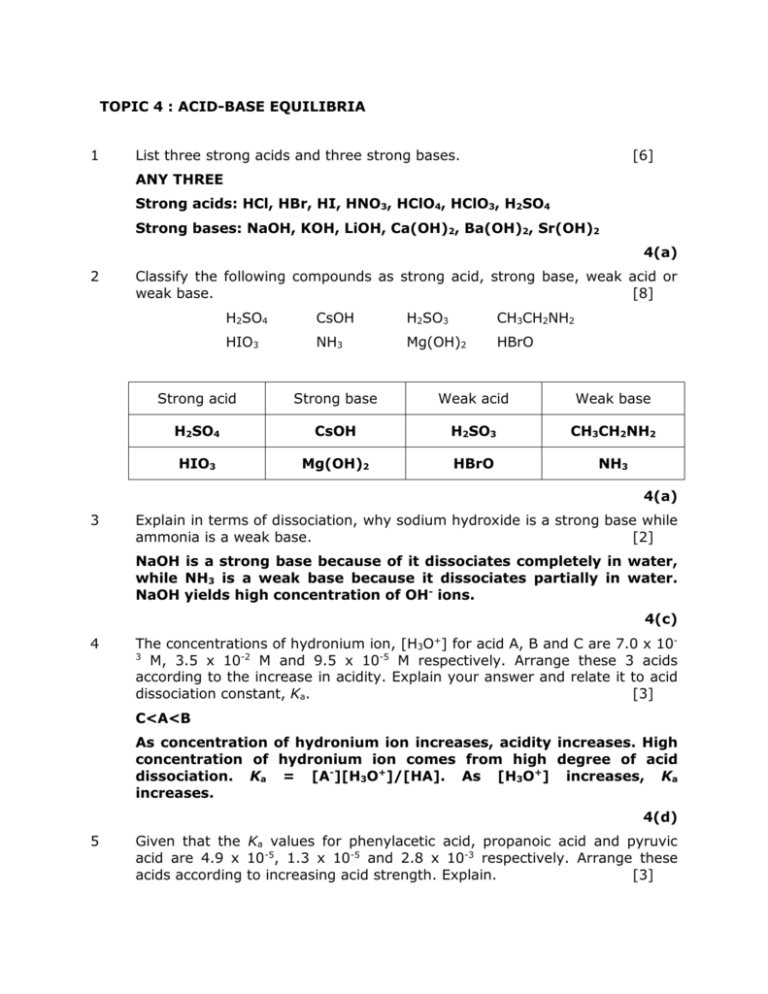
Topic 4 Acid Base Equilibria List Three Strong Acids And Three


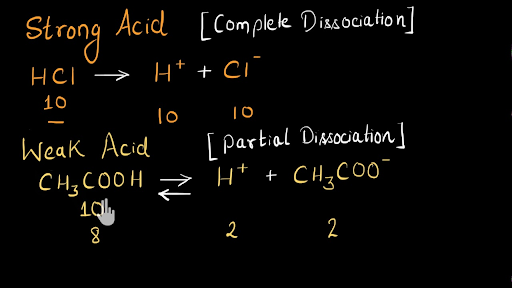
Comments
Post a Comment